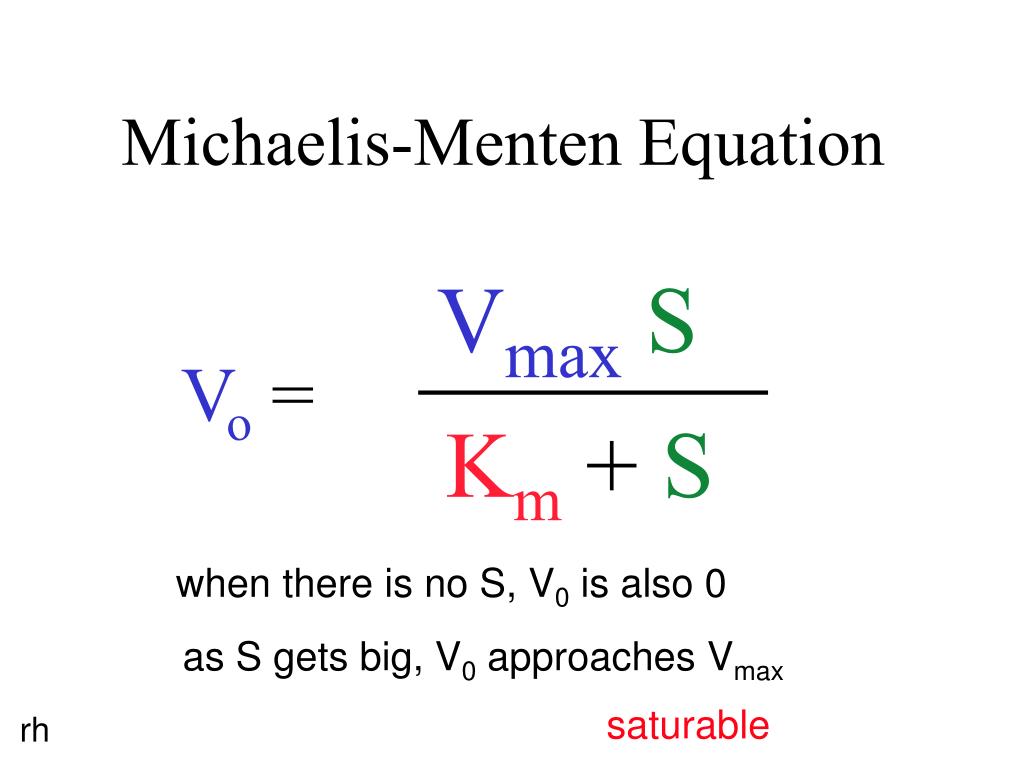
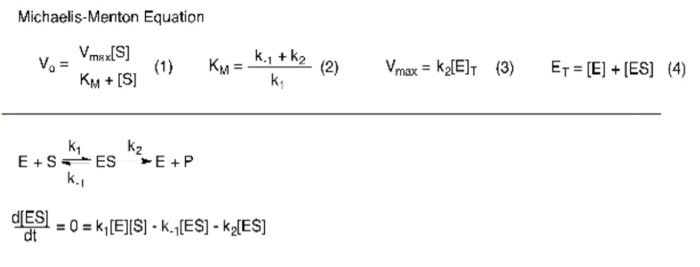
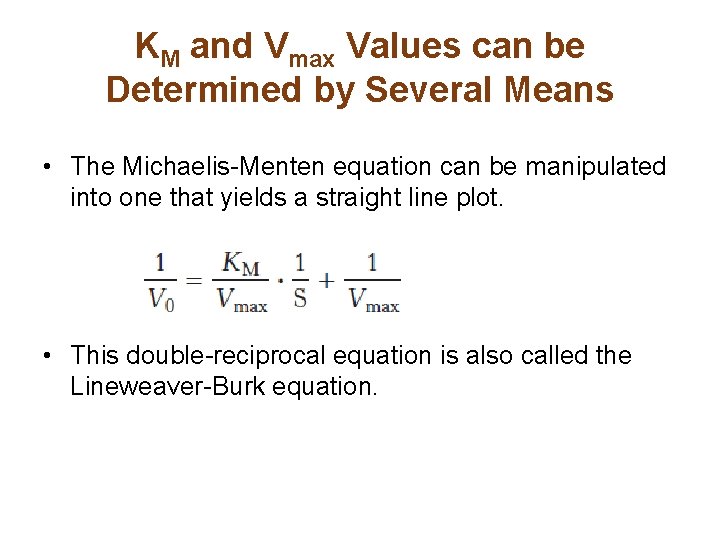
In the derivation of the MichaelisMenten equation based on the following reaction k1 k2 E S ES E P k_1 the Michaelis constant KM is defined under the approximation Under the approximation, the Michaelis constant KM is an estimation oft Enzymesubstrate binding affinity Ks (=k_7 /k7 )Looking at the equation, one can readily see that the velocity of the reaction, V, is dependent on the substrate concentration, SIn fact, the MichaelisMenten equation is a rational functionAs rational functions can be difficult to work with graphically, the MichaelisMenten equation can be transformed into a linear equation by taking the reciprocal of both sides as, The MichaelisMenten equation was derived by Leonor Michaelis and his graduate student Maud Menten in 1913, based on work by Victor Henri, and is applicable only to simple enzyme kinetics in which there is only one substrate that is changed immediately to a product during the reaction without forming any intermediate compound, the enzyme in question shows
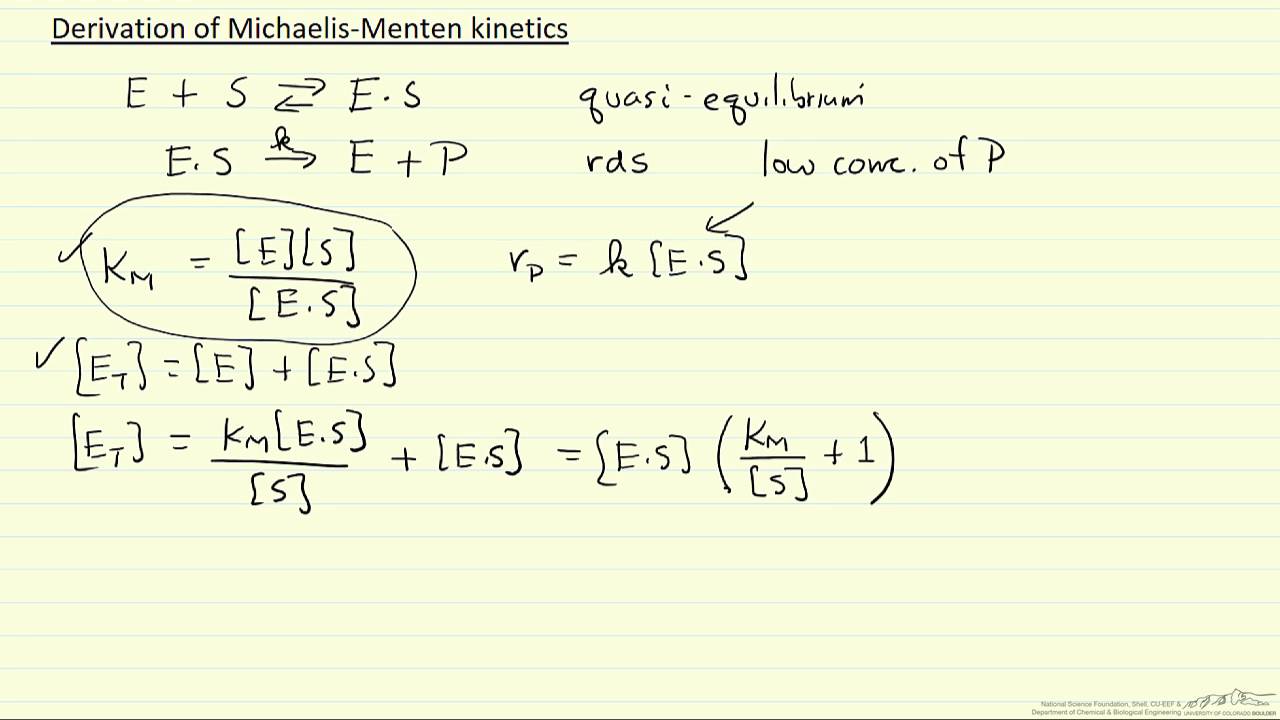
Derivation Of Michaelis Menten Kinetics Youtube
What is km michaelis constant
What is km michaelis constant-#$ SSS SSSS ES ES EP k1 Catalysis k 2 k2 Binding k 1 ES EP = ˇ˙˝ S ˘ˇSWhen S>>Km, V0=Vmax S/ S, this means that the reaction is always catalyzed at full speed and the enzyme cannot be fine tuned by the cell When S
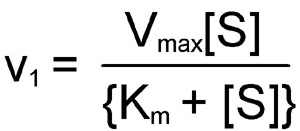



Enzyme Kinetics And Diagnostic Uses Of Enzymes The Medical Biochemistry Page
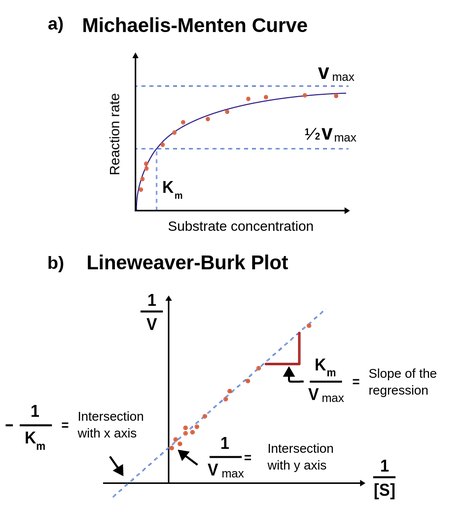
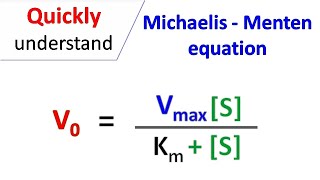
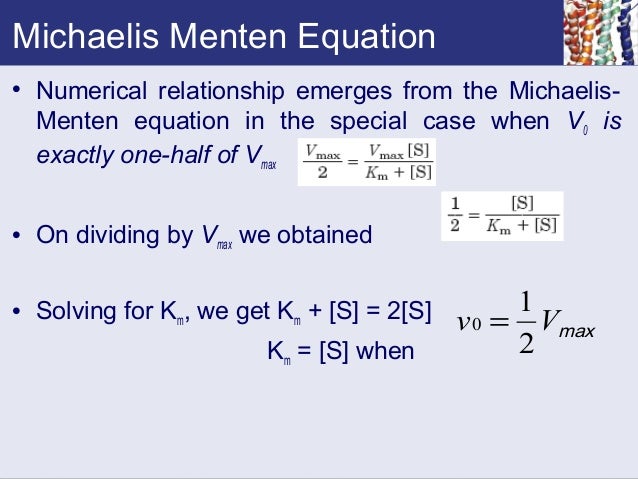
MM equation Vo = Vmax So LB eqn 1/Vo = Km/Vmax(1/So) 1/Vmax Km So 9 How does the MichaelisMenten equation explain why the rate of an enzymecatalyzed reaction is proportional to the amount of enzyme?Km (Michaelis Constant) Km is a parameter of the MichaelMenten equation often called the Michaelis constant or ED50 It is in the same scale as the C values In fact, Km is that C value that results in a velocity of Vmax/2 You may enter a single value or multiple values Michaelis Menten equation derivation and use The MichaelisMenten Model is based on a kinetic concept, expressed in the following enzyme equation E S ⇄ ES → E P Where E stands for Enzyme;
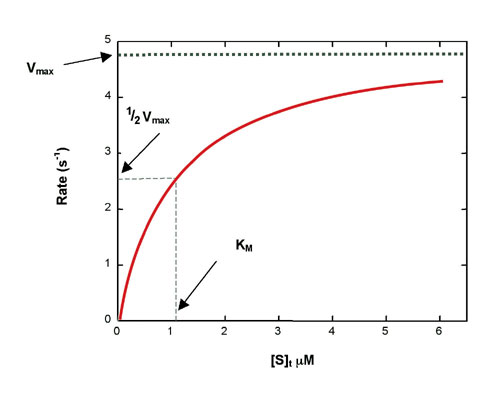
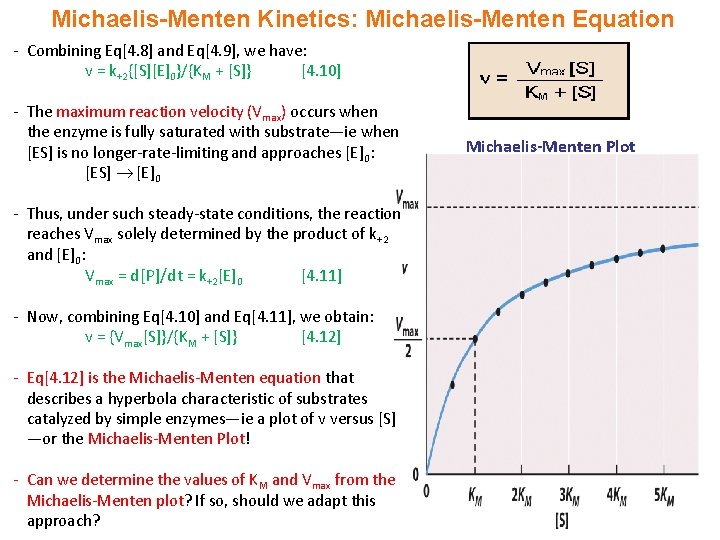
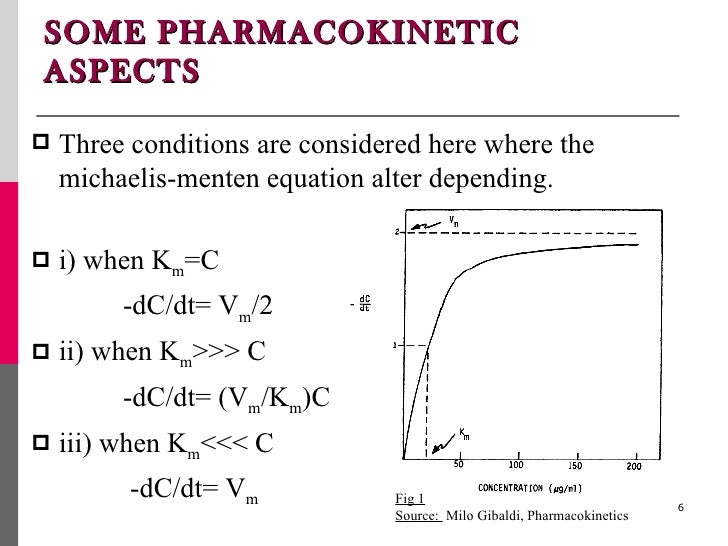
Rewritten into the familiar form of the MichaelisMenten equation (eq 14) S S m max K V v (14) Next, we imagine what happens when Km > > S as follows in eq 15 S S m max k K V v (15) Since k = Vmax/ Km in eq 15, we refer to Vmax/ Km asModel associated with the Michaelis Menten equation is 𝑉𝑉= C(Vmax) C Km 𝜀𝜀 where ε represents normally distributed errors with zero mean and constant variance 𝜎𝜎2 It provides estimates, confidence intervals, and statistical hypothesis tests based on this assumption The method is documented in the Therefore, to calculate V max and Km, it is typical to transform the MichaelisMenten equation by taking the reciprocal of both sides You can rearrange Eq 4 to get If you look carefully at Eq 5, you will see that it is the equation of a line (y=mxb), where y=1/V, m= Km/V max, x=1/S, and b=1/V max
In the MM equation above Vmax = k 2Eo In the experiment of Vo vs Eo, So is held constant so all other terms, So, Km, are constant The plot provides a useful graphical method for analysis of the Michaelis Menten equation Taking the reciprocal gives V = reaction velocity (the reaction rate), Km = MichaelisMenten constant, Vmax = maximum reaction velocity S is the substrate concentration 17 Effect of enzyme concentration on reaction rate 18 The MichaelisMenten equation can then be rewritten as V= Kcat Enzyme S / (Km S) Kcat is equal to K2, and it measures the number of substrate molecules "turned over" by enzyme per second The unit of Kcat is in 1/sec The reciprocal of Kcat is then the time required by an enzyme to "turn over" a substrate molecule



Using Isothermal Titration Calorimetry




Michaelis Menten
A single equation for the velocity of the reaction, V = dS/dt † V = dS dt = VmaxS Km S (12) where † Vmax = k2ETotal Km = k1 k2 k1 The function V = Vmax!S Km!!S is the MichaelisMenten hyperbola Km is the value of S when the velocity of the reaction is half its maximum, Vmax, and the slope of the V(S) curve is Vmax/Km Exercise To understand MichaelisMenten Kinetics, we will use the general enzyme reaction scheme shown below, which includes the back reactions in addition the the forward reactions (2) E S → k 1 E S → k 2 E P (3) E S ← k 3 E S ← k 4 E P The table below defines each of the rate constants in the above scheme Table 1 ModelNinja Nerds,Join us in this video where we discuss the michaelis menten equation***PLEASE SUPPORT US***PATREON https//wwwpatreoncom/NinjaNerdScience***



Ii Protein Biochemistry 2 6 Enzyme Kinetics 2
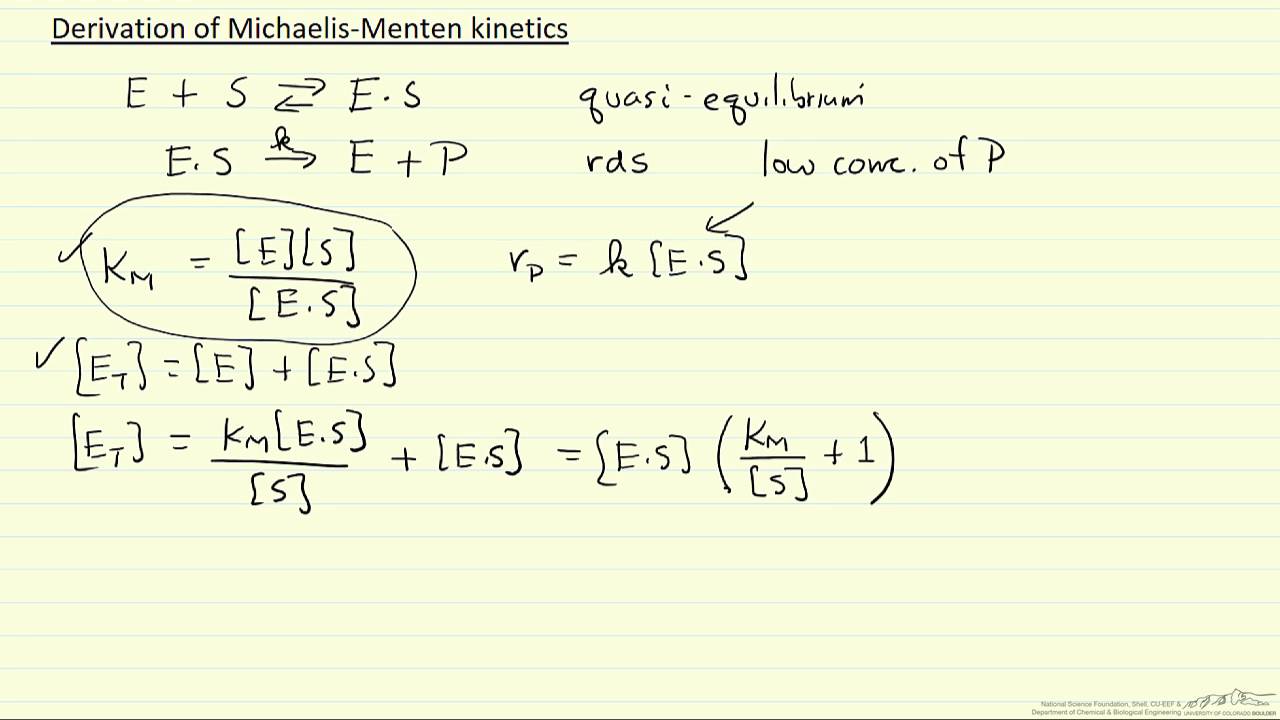



Derivation Of Michaelis Menten Kinetics Youtube
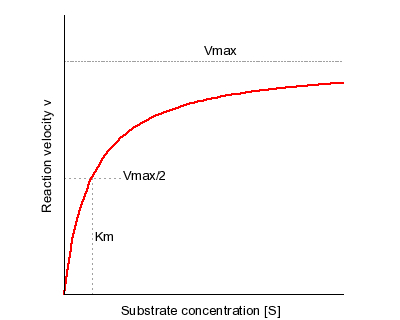
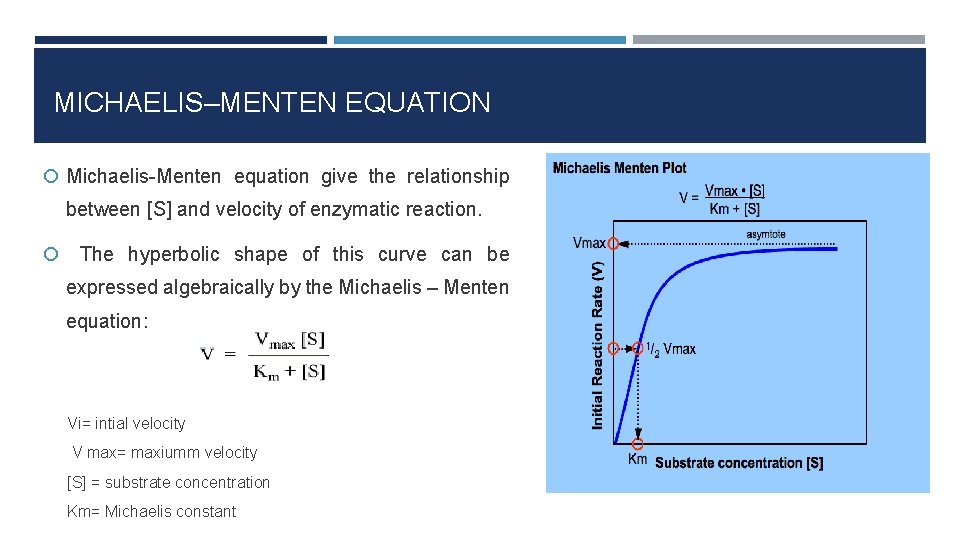
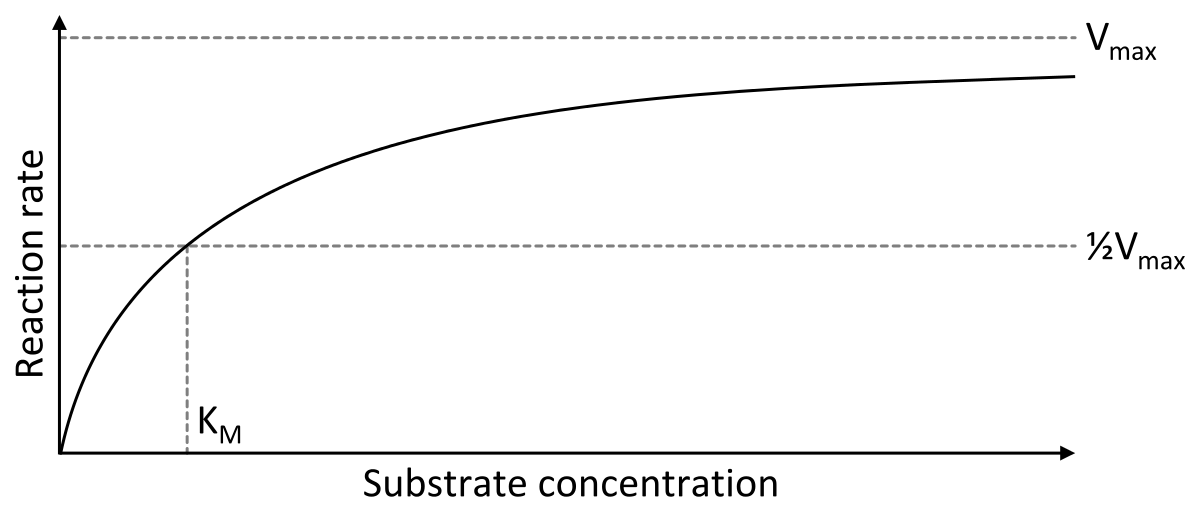
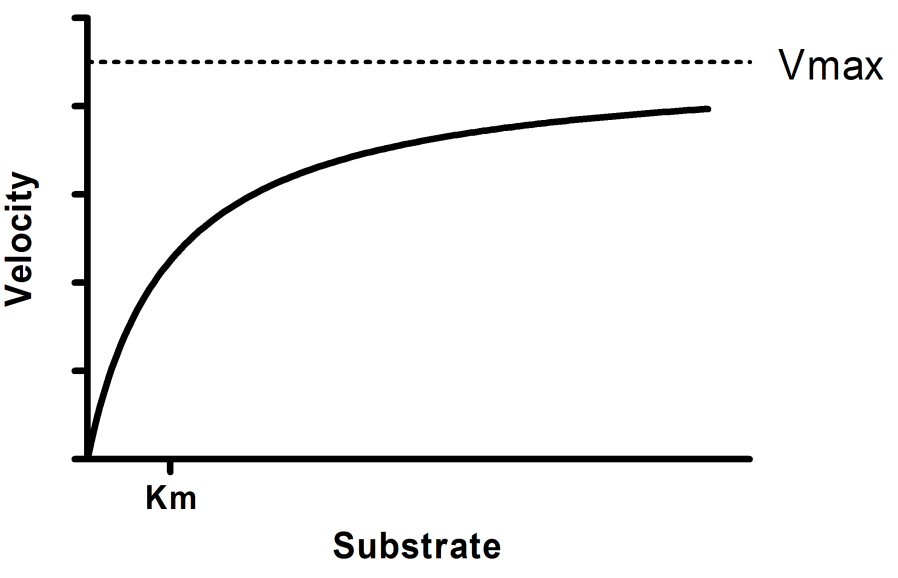
The MichaelisMenten equation can be expressed as The velocity is therefore proportional to the enzyme concentration , not inversely so is also referred to as the turnover number As the substrate concentration keeps increasing, then we end up with a steady state in which all the enzyme is boundThe MichaelisMenten equation forthis system is Here, Vmaxrepresents the maximum velocity achieved by the system, at maximum (saturating)substrate concentrations KM(the Michaelis constant;sometimes represented as KSinstead) is the substrateconcentration at which the reaction velocity is 50% of the VmaxEquation (11), the MichaelisMenten equation, describes the kinetic behavior of an enzyme that acts according to the simple model (1) Equation (11) is of the form y = ax/(b x) (does this look familiar?) This is the equation of a rectangular hyperbola, just like the saturation equation for the binding of dioxygen to myoglobin



4 Basic Concepts Of Michaelis Menten Kinetics The Chegg Com




Structural Biochemistry Enzyme Michaelis And Menten Equation Wikibooks Open Books For An Open World
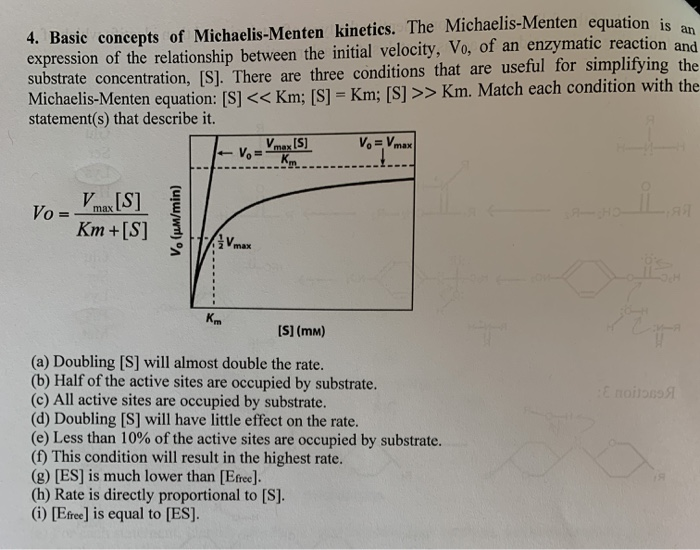
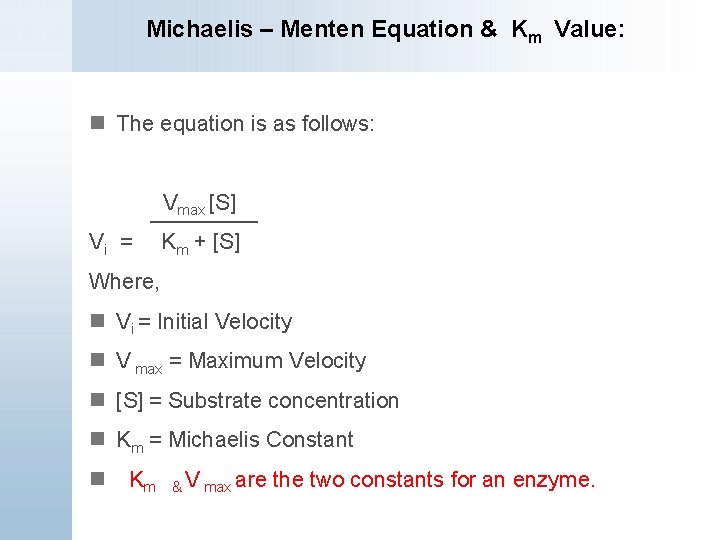
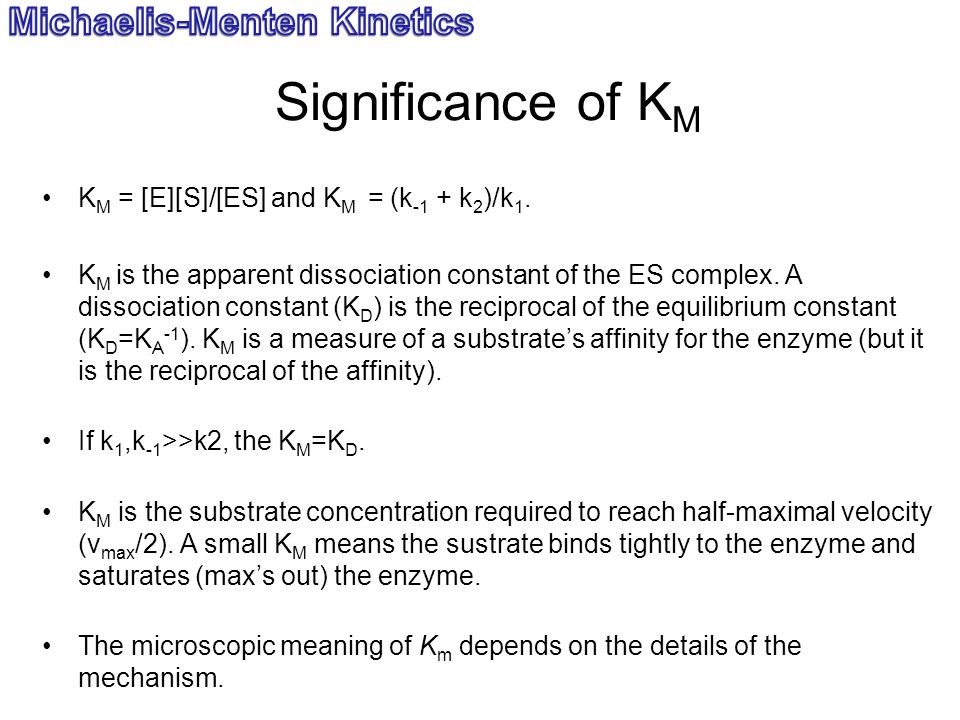
S = Substrate concentration Km = Michaelis constant Although Km values are more or less constants for particular enzymesubstrate systems, but these may vary slightly with pH, temperature, ionic strength and also with types and amount of coenzymes when required for the reaction The values of Km are measured in terms of molarity The MichaelisMenten equation is the most widely known model in enzyme kinetics Where v0 is the initial reaction rate, S is the substrate concentration, Km is the Michaelis constant, and Vmax is the maximum reaction rate The Michaelis constant describes the kinetics of substrate/enzyme bindingWhat does the Michaelis Menten equation tell us?



Michaelis Menten Kinetics




Enzyme Kinetics Given Km Find Substrate Concentration At A Certain Velocity Chemistry Stack Exchange
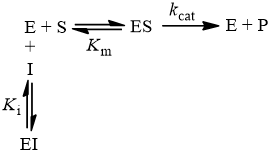
K m is the MichaelisMenten constant which shows the concentration of the substrate when the reaction velocity is equal to one half of the maximal velocity for the reaction It can also be thought of as a measure of how well a substrate complexes with a given enzyme, otherwise known as its binding affinityIn the presence of 2 × 103 M competitive inhibitor that is bound to the enzyme with a K i of 103 M, the apparent K M (K app M) will be equal to K M × (1 I/K i), or 3 × 104 M Substitution of these values into equation 23 gives V 0 = V max /4, when S = 104 M The presence of the competitive inhibitor thus cuts the reaction rate in half at this substrate concentrationP stands for Product The enzyme binds with the substrate, and that connection results in a product forming



Student Consult Ver 2 3



Enzymes Dr Manjula Shantaram Ph D Biochemistry Dept
The Michaelis–Menten equation (Eqn (4)) is the rate equation for a onesubstrate enzymecatalyzed reaction 38 This equation relates the initial reaction rate (v 0), the maximum reaction rate (V max), and the initial substrate concentration S through the Michaelis constant K M —a measure of the substratebinding affinity• MichaelisMenten Equation • Vo, Km, Vmax, Kcat • LineweaverBurk Plot Vmax and Km can be determined by measuring the rate of the reaction at different S if an enzyme operates my MichaelisMenten kinetics Transformation of the MichaelisMenten equation (ie taking the reciprical of both sides) 1/V = 1/Vmax (Km / Vmax x 1/S)MichaelisMenten equation The ratio of kcat to K m can be used to describe an enzyme's catalytic efficiency We also note that kcat Km =k1 k2 k−1 k2 k 1 is the on rate for binding The efficiency of catalysis cannot be greater than the "efficiency" of collisions k 2 / (k1 k 2) describes the fraction of all encounters between E




Integrated Form Of Michaelis Menten Equation Download Scientific Diagram
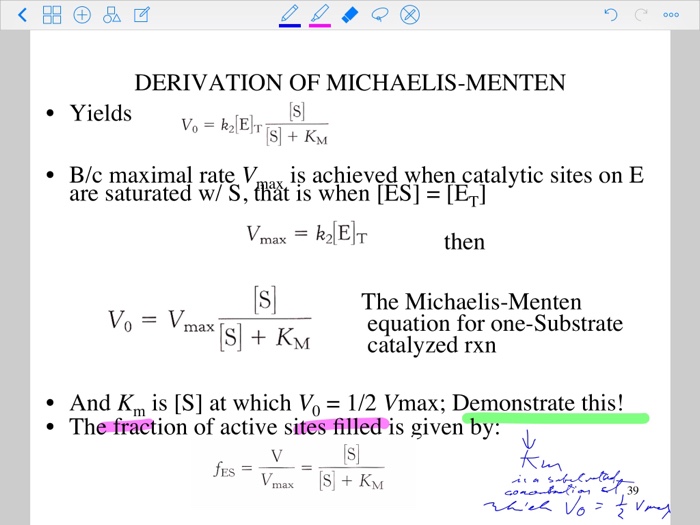



Derivation Of Michaelis Menten Yields B C Maximal Chegg Com
Let us examine both the equations and their plots The MichaelisMenten (MM) plot is called a rectangular hyperbola while the Hill equation plot is called a sigmoid These are just some technical terms to name the different nature of the plots butY = Vmax*X/(Km X) Interpret the parameters Vmax is the maximum enzyme velocity in the same units as Y It is the velocity of the enzyme extrapolated to very high concentrations of substrate, so its value is almost always higher than any velocity measured in your experiment Km is the MichaelisMenten constant, in the same units as X It is the substrate concentration needed to achieve a halfmaximum enzyme velocityMichaelisMenten kinetics are based on the assumptions that A the forward reaction rate (k2) for ES > E P is much greater than the reverse reaction rate, so the reverse reaction is negligible, and B formation and decay of ES is in steady state, thus dES dt = 0 = k ES1 k 2 ES k ES1 formation decay This equation




Team Astws China Model Igem Org
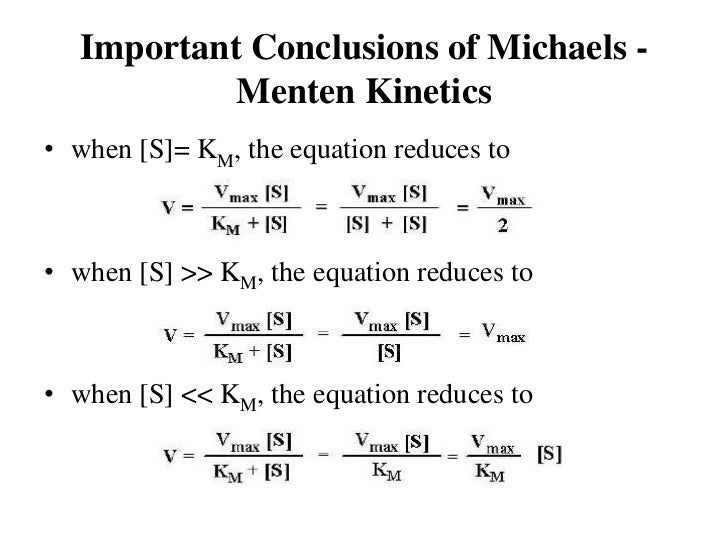



Enzyme Kinetics
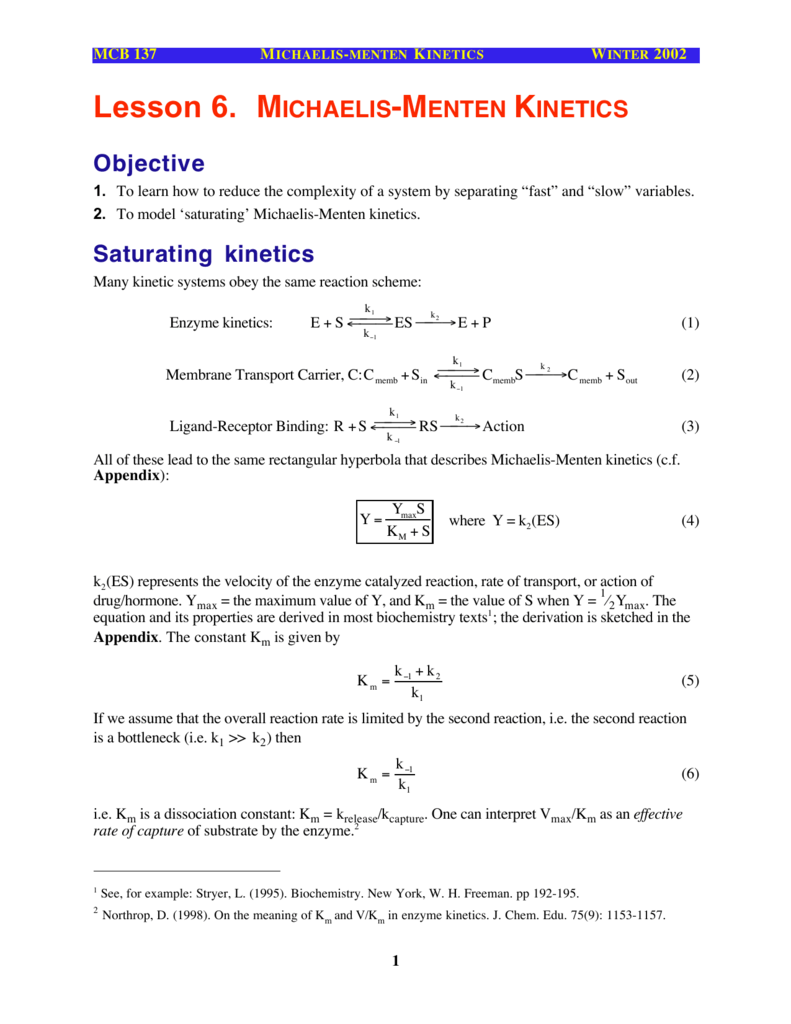
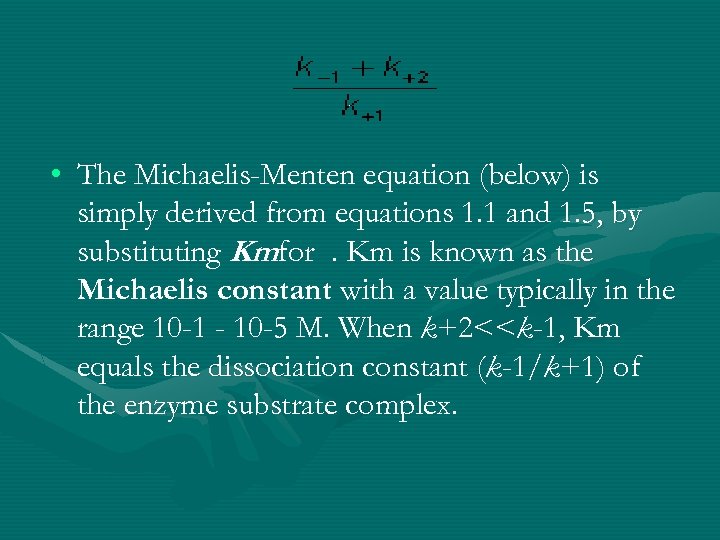
The equation is nonlinear where V = initial velocity of the reaction, Km = Michaelis constant, Vmax = maximum initial velocity, and S = substrate concentration Open AccessThis article is licensed under a Creative Commons Attribution 40 International License, which permits use, sharingThe MichaelisMenten equation a The relationship of substrate concentration to velocity for many enzymes may be described by equation (2), where v is the initial velocity of the reaction, Vmax = k3ET, and Km = (k2 k3)/k1ET is the total E present It should be noted that capital V isDeriving the Michaelis – Menten Equation For this model, let v be the initial velocity of the reaction So in the steady state, k –1 ES k cat ES = k 1 E S (3) To simplify (4), first group the kinetic constants by defining them as K m K m = (k –1 k cat )/k 1 (5)




Example Data Demonstrating Calculation Of Michaelis Menten Constant K Download Scientific Diagram
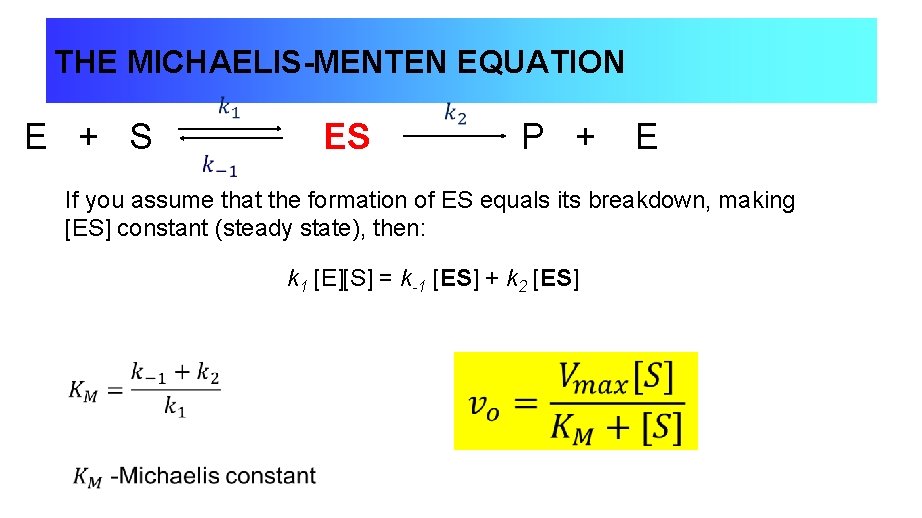



Lecture 2 Enzyme Kinetics General Principles Of Catalysis
In enzyme kinetics, Michaelis–Menten equation is a mathematical equation that relates velocity of enzyme V0, maximum velocity Vmax and Km It explains both,S stands for Substrate;MichaelisMenten equation 27 V = Vmax S/ Km S;
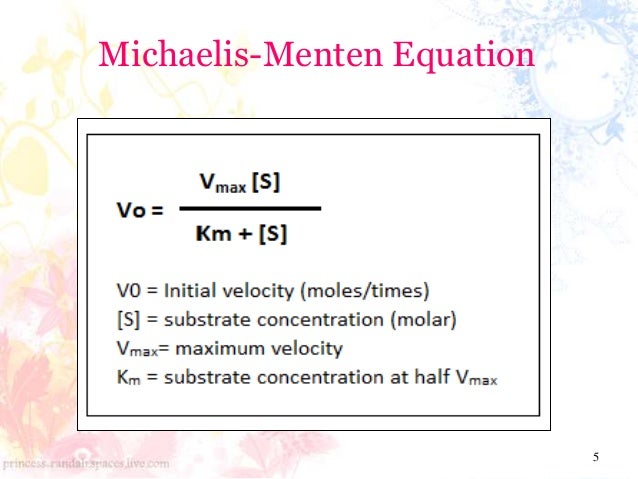



Enzyme Kinetics
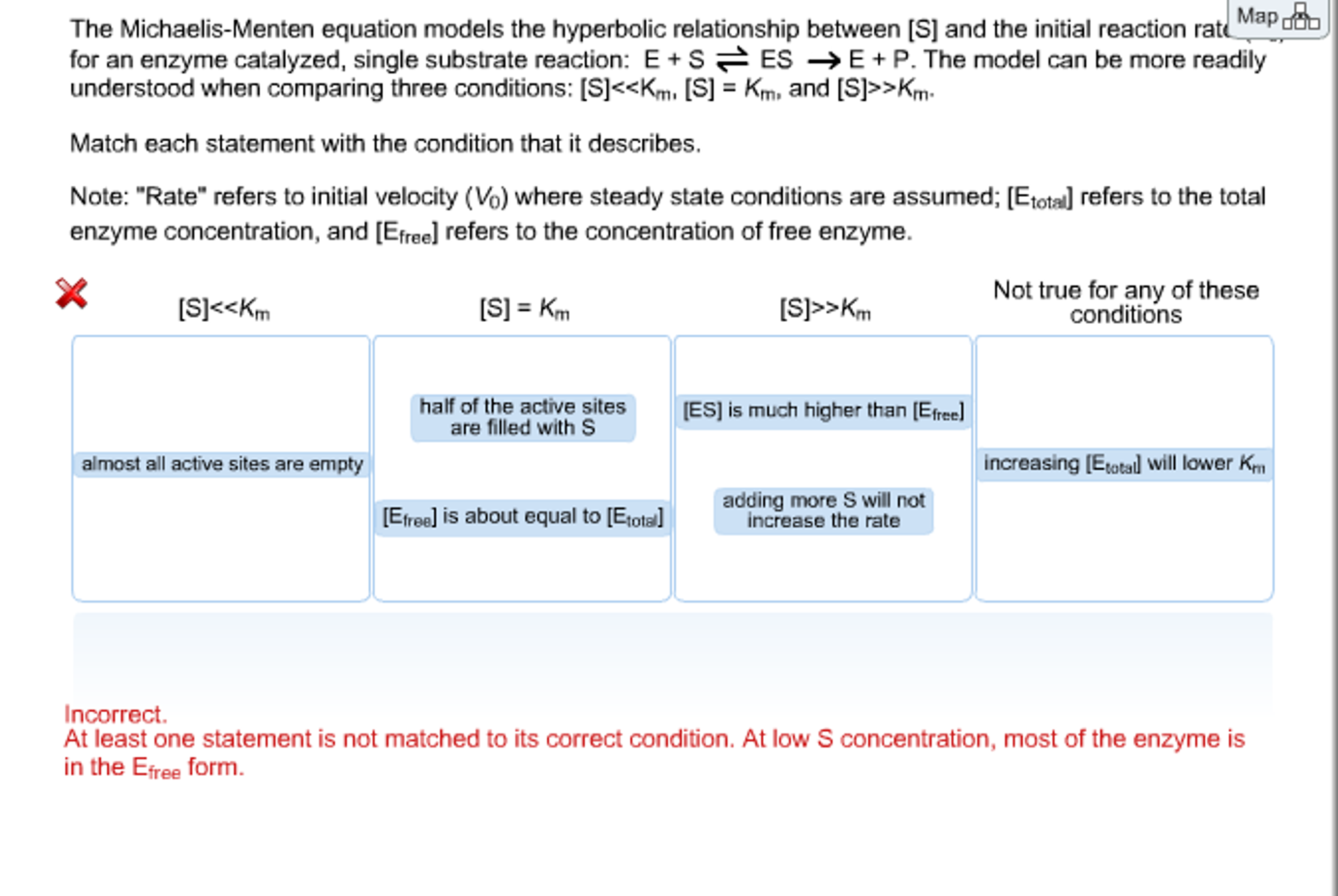



The Michaelis Menten Equation Models The Hyperbolic Chegg Com
The relationship is defined by the MichaelisMenten equation v = Vmax / (1 (Km/S)) I t is difficult to fit the best hyperbola through the experimental points, and difficult to determine Vmax with any precision by estimating the limit of the hyperbola at infinite substrate concentrationA MichaelisMenten equation is also applicable to the condition where E is present in large excess, in which case the concentration E appears in the equation instead of S The term has sometimes been used to describe reactions that proceed according to the scheme in which case Km = (k1 kcat)/k1 (BriggsHaldane conditions)The MichaelisMenten equation describes how the initial rate of an enzymecatalzyed reaction varies as the concentration of the substrate changes The rate of the reaction is given as Vo = VmaxS/(Km S) In the equation, Vmax is the maximum r



Structural Biochemistry Enzyme Michaelis And Menten Equation Wikibooks Open Books For An Open World




Enzyme Kinetics Michaelis Menten Equation The Bumbling Biochemist
• The MichaelisMenten equation describes the kinetic behavior of many enzymes • This equation is based upon the following reaction S → P k 1 k 2 E S ↔ ES → E P k1 k 1, k1 and k 3 are rate constants for each step To derive the equation, they made 2 assumptions 1 The reverse reaction (P → S) is not considered because the MichaelisMenten equation Interactive graph The interactive graph provided below allows for a good understanding of the MichaelisMenten equation, how the reaction velocity changes as a function of the substrate concentration, and how changes in Vmax and Km alter the shape of the graph Enter appropriate numerical values for the Maximum• MichaelisMenten Equation • Vo, Km, Vmax, Kcat • LineweaverBurk Plot Vmax and Km can be determined by measuring the rate of the reaction at different S if an enzyme operates my MichaelisMenten kinetics Transformation of the MichaelisMenten equation (ie taking the reciprical of both sides) 1/V = 1/Vmax (Km / Vmax x 1/S)



Michaelis Menten Kinetics And Briggs Haldane Kinetics



Www Chem Purdue Edu Courses Chm333 Spring 13 Lectures Spring 13 lecture 15 Pdf
The Michaelis Menten kinetic equation is used for determining the kinetics of enzymecontrolled reactions, where the biochemical reaction is assumed to be involving a single substrate MichaelisMenten kinetics allows the computing of the rate of the reaction (V 0 ), substrate concentration S, Michaelis constant (K m ), and the maximum rate Where, KM = Michaelis constant = (k1 k2)/k1)M, K m is negligible, and the equation simplifies to) ˇ˙˝ ˛T ˜ˇ˙˝ = ET S S = ET Substituting V max in to the rate equation gives the MichaelisMenten equation !" =!



1
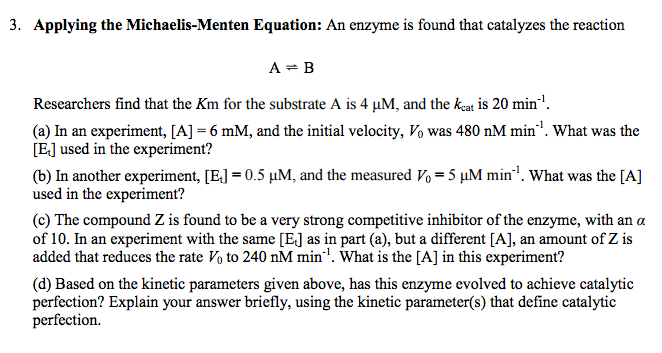



3 Applying The Michaelis Menten Equation An Enzyme Chegg Com
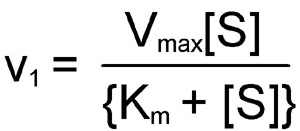



Enzyme Kinetics And Diagnostic Uses Of Enzymes The Medical Biochemistry Page
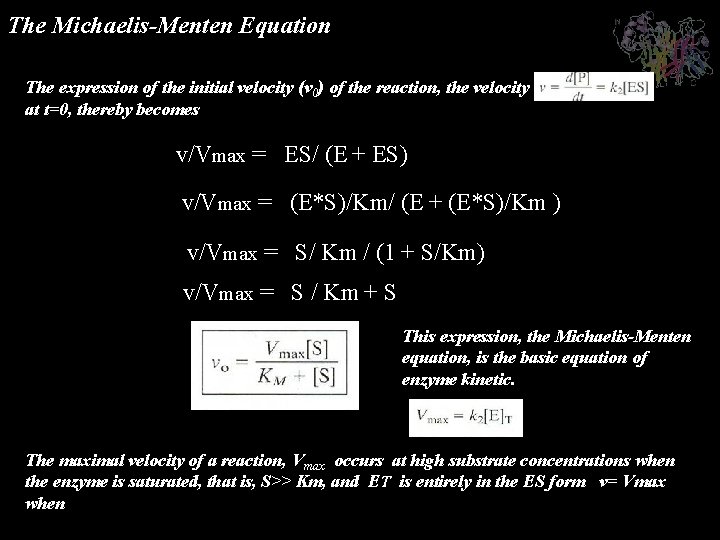



The Michaelismenten Equation Et E Es V Vmax




The Michaelis Menten Equation Is One Of The Chegg Com




Michaelis Menten Rate Equation Theory And Derivation Youtube



Www Chem Purdue Edu Courses Chm333 Spring 13 Lectures Spring 13 lecture 15 Pdf



The Michaelis Menten Equation Curve For Enzyme Activity On Y Axis And Download Scientific Diagram
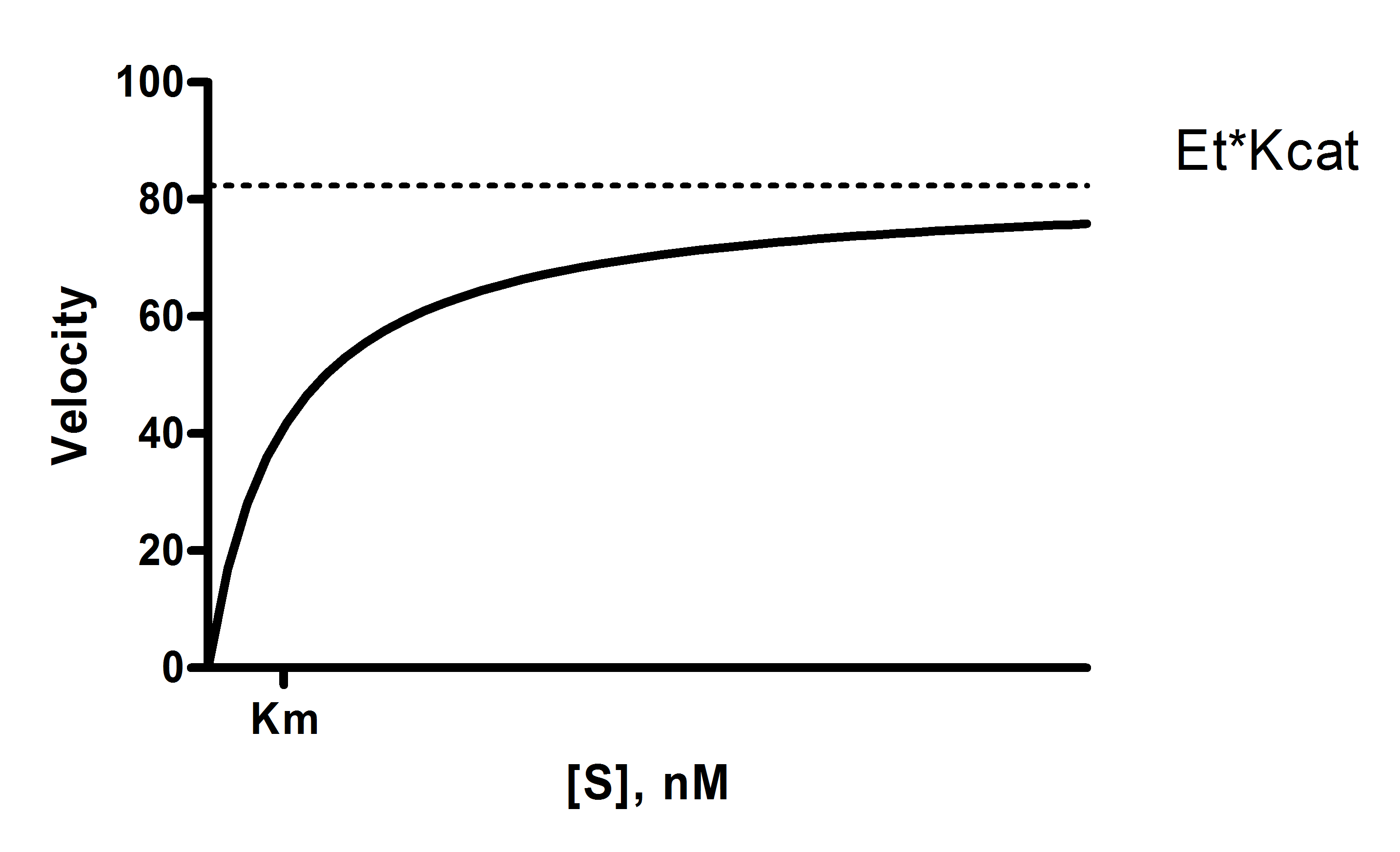



Graphpad Prism 9 Curve Fitting Guide Equation Determine Kcat



4 2a Derivation Of The Michaelis Menten Equation Chad S Prep




044 Michaelis Menten Equation Youtube
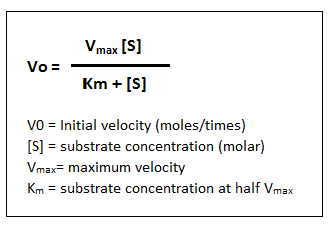



Biology Notes For A Level 21 Michaelis Menten Equation And Immobilising An Enzyme



Chem 440 Special Topic




Inhibited Enzyme Kinetics Inhibitors May Bind To Enzyme And Reduce Their Activity Enzyme Inhibition May Be Reversible Or Irreversible For Reversible Ppt Download




Scielo Brasil Accuracy Of Analytical Numerical Solutions Of The Michaelis Menten Equation Accuracy Of Analytical Numerical Solutions Of The Michaelis Menten Equation
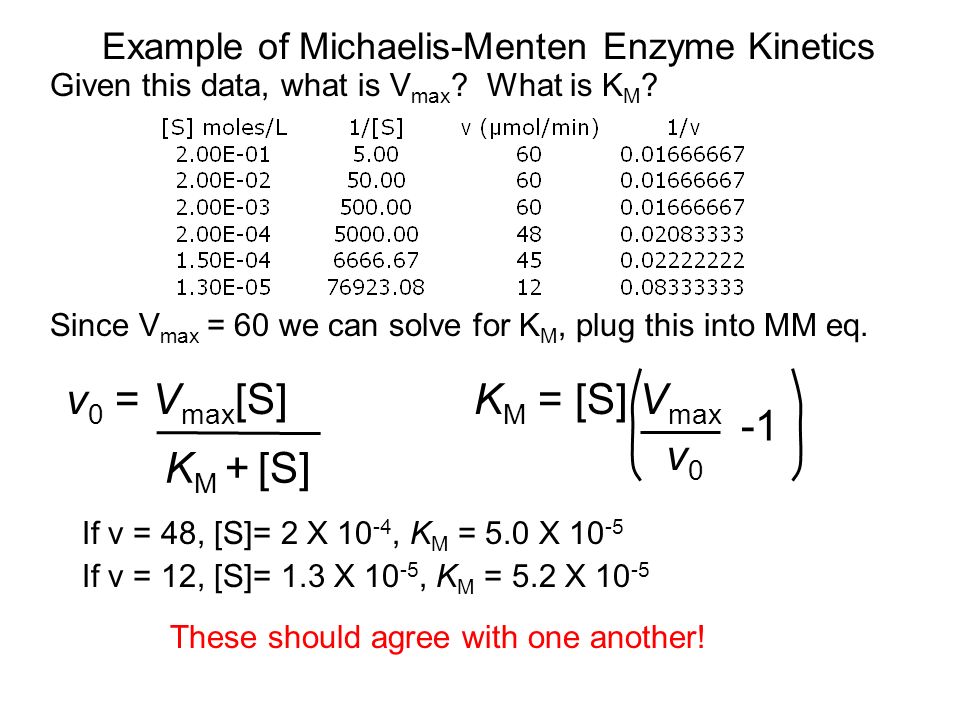



Lecture 15 Enzymology Today Howard Salis 148 Baker 3pm Extra Credit Seminar Assignments Due Monday Ppt Video Online Download




Derivation Of Michaelis Menten Equation Youtube




Biochemistry I Michaelis Menten Problem 2 Youtube




Michaelis Menten Plot Estimating Km Youtube



Km Labster Theory




Michaelis Menten Equation Example 2 Youtube



Run Edu Ng Directory Oermedia Pdf



Michaelis Menten Equation Biochemistry Lecture Slides Docsity



Cours Equation Henri Michaelis Menten Briggs Haldane Constante Catalytique Vmax Km Lineweaver Burk Dixon Hanes Woolf Enzymologie Enseignement Et Recherche Biochimie Emmanuel Jaspard Universite Angers Biochimej



Ppt Michaelis Menten Equation Powerpoint Presentation Free Download Id 121



322 h Exp 7 The Effect Of Substrate




Hanes Woolf Plot To Identify Km And Vmax Which Were Used In The Download Scientific Diagram



Www Chem Purdue Edu Courses Chm333 Spring 13 Lectures Spring 13 lecture 15 Pdf




The Michaelis Menten Equation Kcat Km



Chem 440 Special Topic



Michaelis Menten Kinetics Wikipedia




Kinetic Analysis Michaelis Menten Equation



Run Edu Ng Directory Oermedia Pdf



1
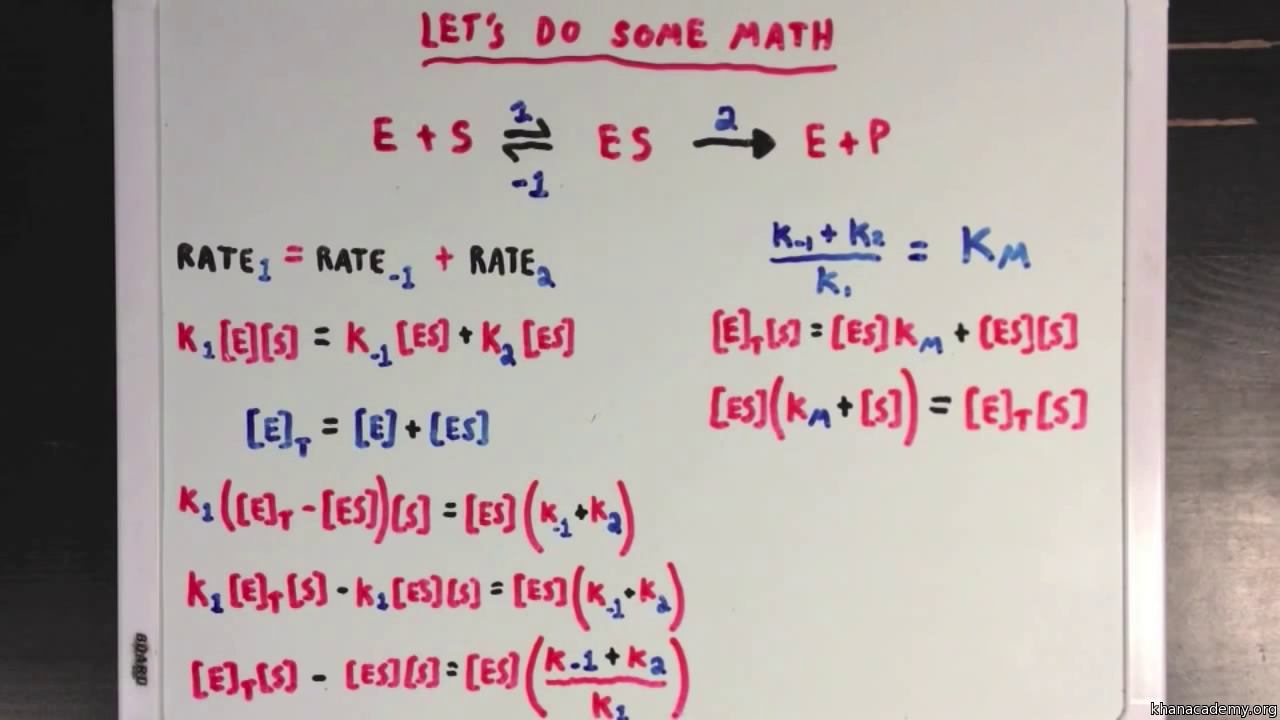



Steady States And The Michaelis Menten Equation Video Khan Academy




Enzyme Kinetics Michaelis Menten Equation The Bumbling Biochemist




What Is The Relationship Between The Hill Equation And Michaelis Menten Kinetics Quora




Michaelis Menten Equation Biolympiads
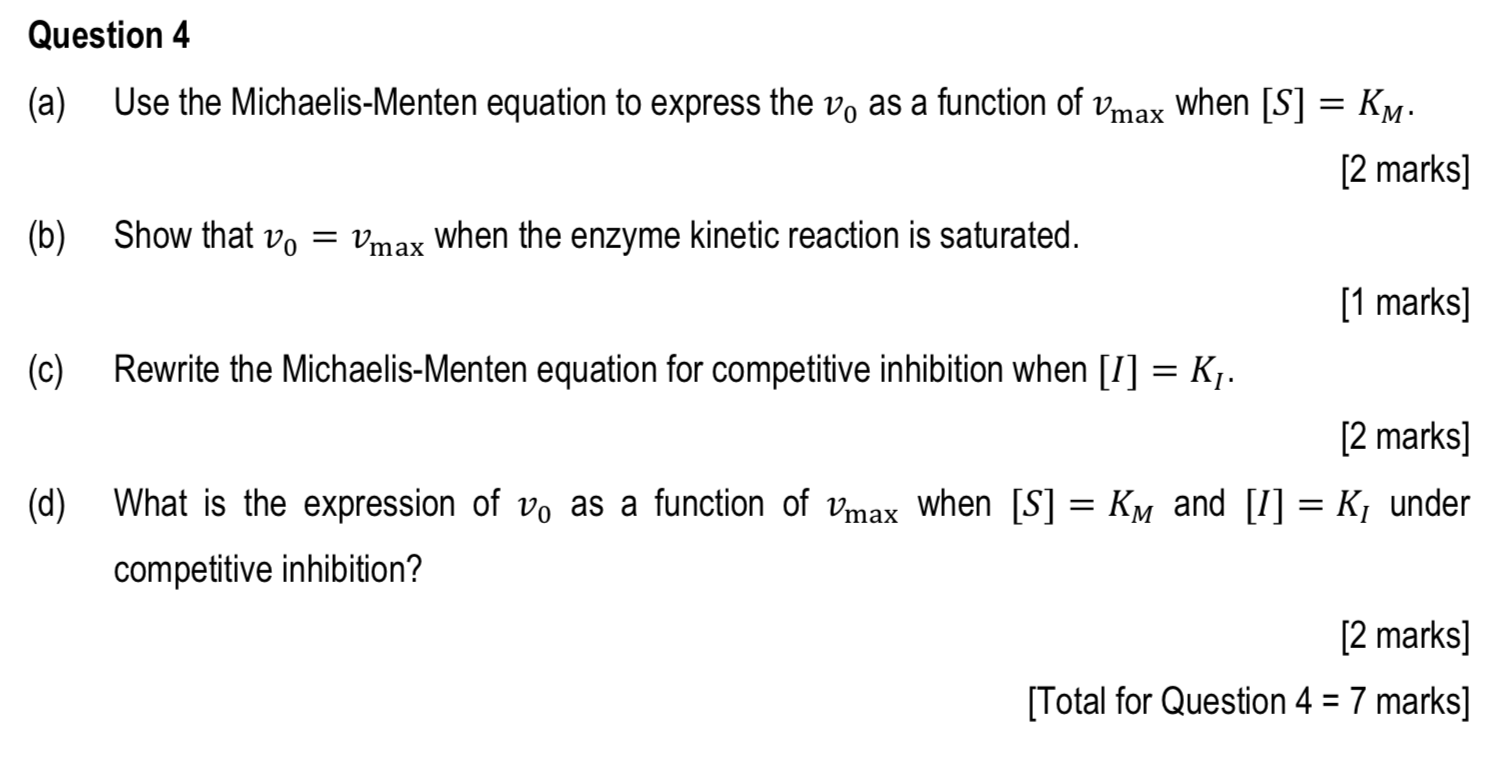



Question 4 Use The Michaelis Menten Equation To Chegg Com
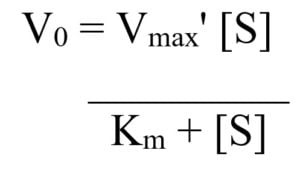



The Michaelis Menten Model Biochemistry Microbe Notes
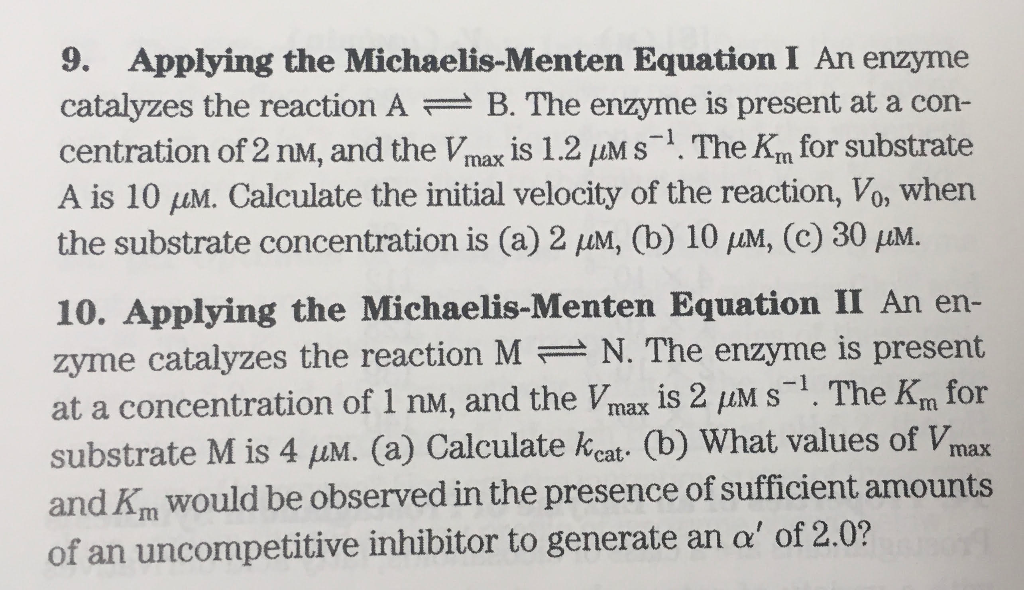



9 Applying The Michaelis Menten Equation I An Enzyme Chegg Com



Blog Archives The Science Snail




Help Online Origin Help Michaelismenten



Description The Michaelis Menten Equation Equation Chegg Com



Lesson 6 Michaelis Menten Kinetics Objective



1




Deriving The Michaelis Menten Equation Youtube




Kinetic Parameters Using Michaelis Menten Equation V Max 1 060 28 Download Scientific Diagram




Michaelis Menten Explained And Derived Youtube




Michaelis Menten Equation Youtube



Content Of Enzyme Technology Why




Developing A Three Dimensional Animation For Deeper Molecular Understanding Of Michaelis Menten Enzyme Kinetics Florjanczyk 18 Biochemistry And Molecular Biology Education Wiley Online Library




Michaelis Menten Rlc Steve 313 Campbell hm313 Michaelis Menten Equation The Ratio Of Kcat To K M Can Be Used To Describe An Enzyme S Estimation Of Vmax And K M From A Direct Pdf Document
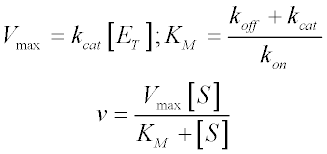



Michaelis Menten Kinetics And Briggs Haldane Kinetics



Michaelis Menten Equation Youtube




Enzyme Kinetics Michaelis Menten Equation The Bumbling Biochemist



Enzyme Kinetics Inhibition And Control Ppt Video Online Download




Structural Biochemistry Enzyme Michaelis And Menten Equation Wikibooks Open Books For An Open World



Michaelis Menten Equation Calculator



Graphpad Prism 9 Curve Fitting Guide Equation Michaelis Menten Model




Lineweaver Burk Plot Wikipedia




Integrated Form Of Michaelis Menten Equation Download Scientific Diagram



Michaelis Menten Kinetics Wikipedia



The Michaelis Menten Equation Models The Hyperbolic Relationship Between S And The Initial Answersbay




Steady States And The Michaelis Menten Equation Video Khan Academy




Michaelis Menten Kinetics Wikipedia
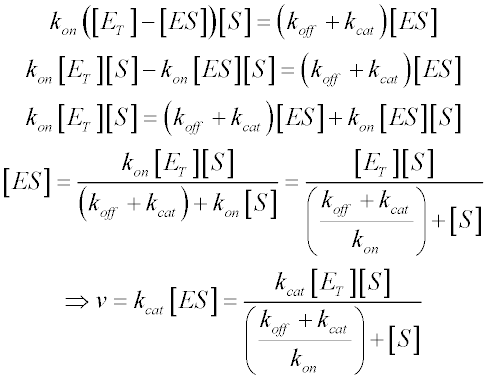



Michaelis Menten Kinetics And Briggs Haldane Kinetics



1
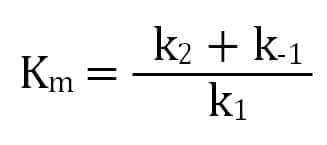



A Quick Primer On Enzyme Kinetics




Michaelis Menten Equation Flashcards Quizlet




Michaelis Menten Equation Justanotherbiochemian




Apparent K M And V Max Values Calculated From The Michaelis Menten Download Table



Chapter 8 Enzymes Basic Concepts And Kinetics 19
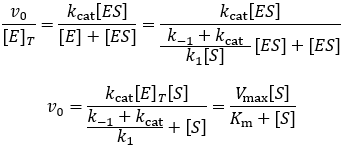



Km Vs Kd The Difference Between Michaelis And Dissociation Constants The Science Snail




Rectangular Hyperbola Plot Of The Michaelis Menten Equation Relating Download Scientific Diagram




The Meaning Of The Michaelis Menten Constant Km Describes A Steady State Biorxiv




Kinetic Analysis Michaelis Menten Equation




Michaelis Menten Equation Interactive Graph Physiologyweb



Enzyme Kinetics




Rmax And Km 26 4 Constants From Michaelis Menten Equation Give Insight Into Qualitative And Quantitative Aspects Of Enzyme Kinetics Indicate If Enzyme Ppt Video Online Download




Michaelis Menten Equation An Overview Sciencedirect Topics



Www Chem Purdue Edu Courses Chm333 Spring 13 Lectures Spring 13 lecture 15 Pdf



0 件のコメント:
コメントを投稿